In accordance with the provisions of Regulation (EU) 2016/679 on the protection of personal data of natural persons (RGPD), we inform you that the personal information you provide will be duly registered and incorporated into the data processing systems under the responsibility of:
Company Name: Euroespes S.A.
Commercial Name: EuroEspes S.A.
Registered Office: Santa Marta de Babio S/N - Bergondo (A Coruña)
CIF / NIF: A15319452
Telephone: 981780505
Fax: 981780511
e-Mail: info@euroespes.com
Registered in the Registry: R.M. of La Coruña, Volume 855, General Section, Folio 202, Page C-1635, Inscription 1ª.
Domain name: euroespes.com
Contact details of the Data Protection Delegate: delegado@euroespes.com
What is epigenetics
It connects genomic and environmental factors affecting health.
What is Epigenetics?
Epigenetics studies the heritable and reversible changes that occur in gene function without modifying the DNA sequence.
Physical and mental health depends not only on the modifications in the DNA code inherited from our ancestors (genetics), but also on the dynamic interaction between our genes and the environment, even if the genetic code is not altered (epigenetics).
Epigenetic changes are reversible and depend on the quality of the interaction between the individual and his environment, and therefore we can act on them to improve our health.

Influence of Epigenetics:
Twin studies
An illustrative example of the influence of epigenetics is seen in studies of monozygotic identical twins. Although they share an identical DNA sequence, both develop a very different predisposition to certain types of diseases.
This is largely explained by the fact that both have been exposed to very different stimuli (diet, social life, hobbies, vices, stress, etc.), and their genomes, although identical, are expressed differently.
What is the importance of epigenetics in medicine?
Epigenetics plays a fundamental role in the development of prevalent diseases (cardiovascular, cancer and brain). Thus, for example, mechanisms such as memory and learning, cognitive impairment associated with aging or behavioral disorders, are largely epigenetically regulated.
Epigenetic biomarkers
EuroEspes is developing a series of epigenetic biomarkers that allow both the detection of diseases and the monitoring of the response to the different treatments provided to the patient:
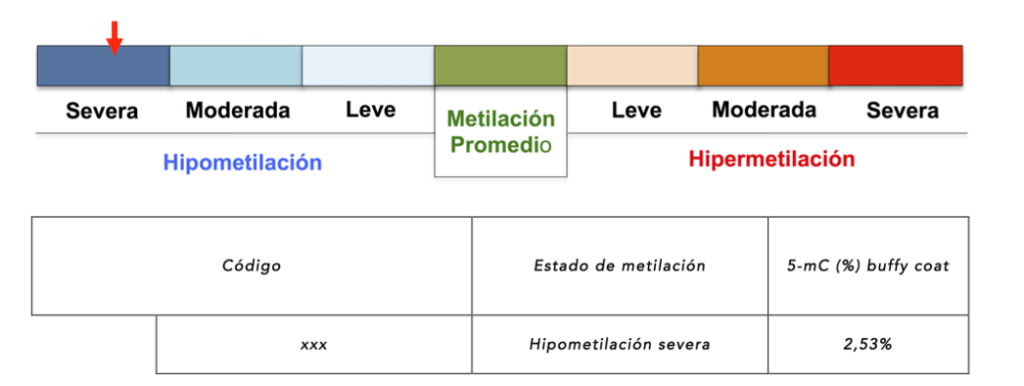
Global DNA methylation
In tumor and neurodegenerative processes there are lower levels of global DNA methylation. Thus, this biomarker makes it possible to detect whether these global methylation levels correspond to those of a healthy person, or whether there are abnormal levels compatible with tumor and neurodegenerative processes. In addition, treatment with AtreMorine, an epinutraceutical that increases Dopamine levels, increases global methylation levels in Parkinson's patients.
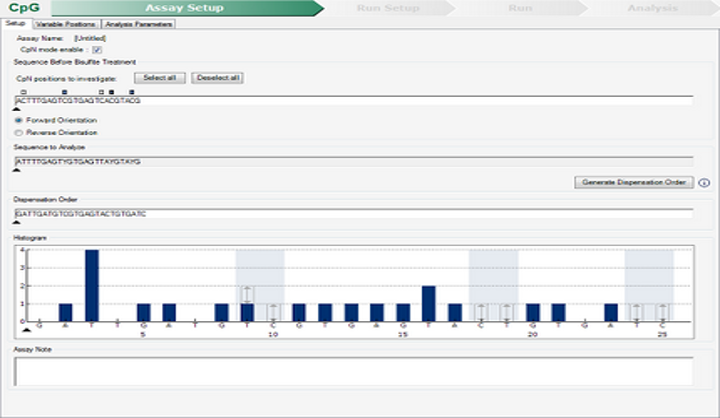
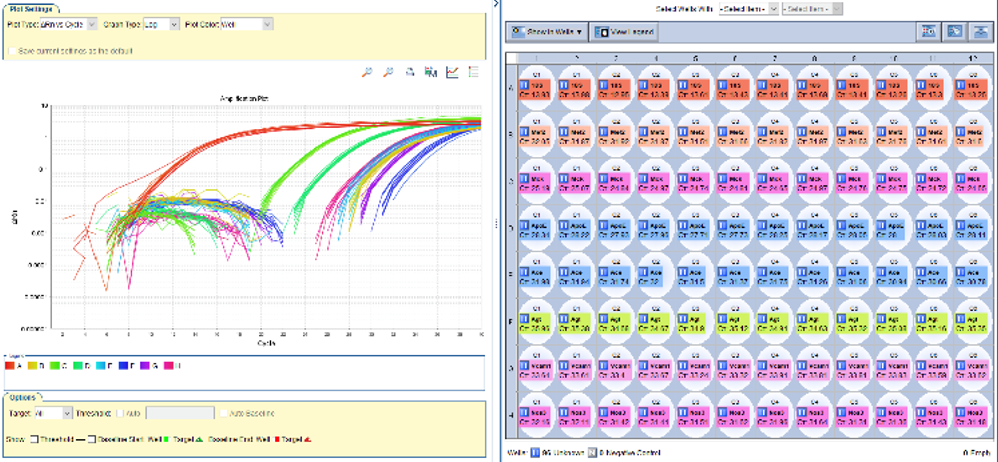
Pyrosequencing
The pyrosequencing technique allows the quantification of methylation levels in certain regions of some genes, allowing the diagnosis of certain pathologies (Parkinson's disease, Alzheimer's disease, colorectal cancer, etc.). For example, Septin 9 methylation is altered in colorectal cancer. The study of Septin 9 methylation levels is a biomarker for screening and early diagnosis of this type of cancer (approved by the FDA and the EU).
Gene expression
Changes in methylation levels often lead to changes in gene expression. Thus, the study of the expression levels of important genes in certain pathologies is also a new biomarker.
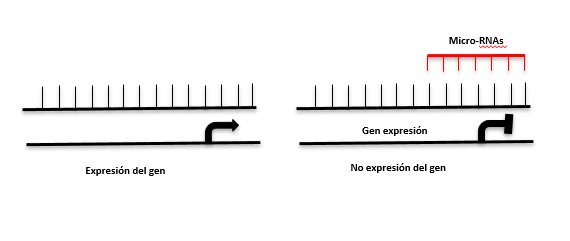
miRNAs
The expression levels of certain miRNAs are associated with different pathologies. For example, an epigenetic signature of 12 miRNAs is a biomarker for the early diagnosis of thyroid cancer and another signature of 8 miRNAs is used for the diagnosis of lung cancer.
PharmacoEpigenetics
The differences between individuals in drug response and metabolism are explained by both the genetics and epigenetics of the individuals.
It has been described, for example, that smoking affects the methylation of the CYP1A1 gene, involved in drug metabolization. In addition, methylation of different genes in tumor processes affects the response to different chemotherapeutic agents. For example, MGMT methylation levels are related to the response to chemotherapy in glioma patients.
Thus, the combination of genetic and epigenetic studies is a fundamental pillar of personalized medicine and allows better treatment of patients, avoiding ineffective or even toxic drugs.
Among the R&D projects of the EuroEspes Medical Epigenetics Department is the study of the epigenetic changes that occur in the genes involved in drug metabolism and their association with the response to these treatments.
If you have any questions or need more information
do not hesitate to contact us.

